Aug. 12, 2022
Snyder Institute researchers discover new approach to healing skin infections and wounds

University of Calgary researchers have identified a promising new approach to treating bacterial skin infections. In a study recently published in Nature, first author Dr. Rachel Kratofil, PhD, and co-senior authors Drs. Paul Kubes, PhD, Justin Deniset, PhD, and their research team show new insight which could lead to advancements in treating bacterial infections and wounds.
“While translating our research from bench to bedside will require many more experiments and involve a model more closely related to human disease, it is exciting that we have made a fundamental discovery that could improve infections and tissue repair in humans, especially hard-to-treat cases,” says Kratofil.
Traditionally, researchers have thought that both neutrophils and monocytes (white blood cells) were recruited to clear bacteria from an infected site on the skin. When these cells work together, they act as the immune system’s first line of defense in our bodies.
However, the new research reveals that monocytes alone are capable of facilitating faster healing of wounds. Monocytes help the healing process by regulating leptin levels and blood vessel growth during wound repair. They also produce ghrelin, a hormone that helps wounds heal more efficiently.
Surprising connection between metabolic hormones and tissue repair
Ghrelin is produced by the stomach when you are hungry, and leptin — also a hormone — is produced by fat cells after you eat a meal and feel full. This balance between ghrelin and leptin has long been understood as critical to metabolism and diet, but until now, has not been known for its connection to immune mechanisms and tissue repair.
Using intravital microscopy, which allows observation of live cells and is a specialization of the Kubes Lab, Kratofil was able to visualize the immune response to Staphylococcus aureus (S. aureus) bacteria in an animal model.
S. aureus is a germ commonly found on skin or in the nose of a healthy body. It can be a catalyst for a wide variety of diseases related to skin and tissue infections such as abscesses or boils. In some cases, the bacteria can lead to severe infections like pneumonia and endocarditis, a life-threatening inflammation of the inner lining of the heart's chambers and valves.
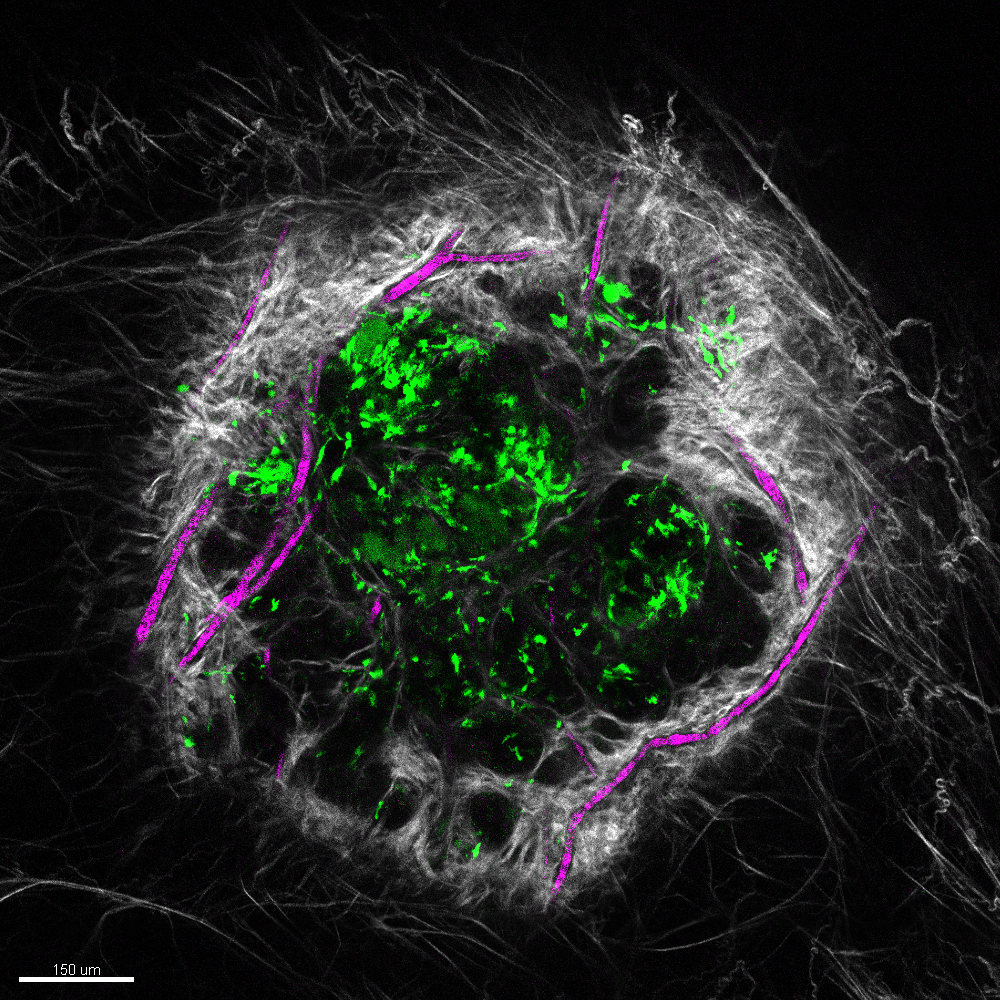
Vascularized wound under the microscope.
Courtesy Rachel Kratofil
After an S. aureus infection, the body recruits those helpful immune cells, neutrophils and monocytes. Neutrophils clear bacteria, while monocytes help repair tissue. In the absence of monocytes there is increased leptin production, leading to blood vessel growth in the infection. The result can be delayed healing and scarring. In contrast, monocytes produce ghrelin at the infection site, which blocks the formation of excess blood vessel growth driven by leptin, leading to tissue repair.
Importance of the study’s results
“This research is important because it indicates a paradigm shift challenging the current thinking that neutrophils and monocytes clear bacteria. Our study elevates the role of monocytes in wound repair,” explains Kratofil.
Principal investigator Kubes and his research team believe this study opens the door to introduce metabolic hormones (ghrelin and leptin) in the fields of immunology and microbiology.
“It will be interesting, for example, to see how ghrelin and leptin respond in other disease models such as sterile injury or cancer, and to learn how these processes are altered when a patient has multiple simultaneous diseases or conditions such as obesity and diabetes,” says Kubes.
Next steps
The next step for the researchers is to better understand the functions of immune cells such as neutrophils during infection. Specifically, they are interested in how neutrophils become cleared from an infection and whether neutrophils take on different functions in addition to clearing bacteria.
This research team’s interdisciplinary work is the product of 133 independent experiments that were carried out in collaboration with the labs of Dr. Keith Sharkey, PhD (Snyder Institute, Hotchkiss Brain Institute (HBI)), Dr. Jeff Biernaskie, PhD, (HBI and Alberta Children’s Hospital Research Institute), and researchers from University Hospital Regensburg, Germany, and Texas A&M University.
Funding for this study was provided by the Canadian Institutes of Health Research.
Rachel Kratofil (@rachelkratofil) is a recent trainee in the Kubes Lab at the Cumming School of Medicine’s (CSM) Snyder Institute for Chronic Diseases. She is a recipient of the Alberta Graduate Excellence Scholarship, the University of Calgary’s Doctoral Scholarship, and the J.B. Hyne Research Innovation Award. She will begin her postdoctoral fellowship at New York University’s Langone Health in the Department of Microbiology in October 2022.
Paul Kubes leads the Infections, Inflammation and Chronic Diseases strategy at the University of Calgary. He is a professor in the Department of Physiology and Pharmacology at the CSM and a member of the Snyder Institute for Chronic Diseases.
Justin Deniset is an assistant professor in the departments of Physiology and Pharmacology and Cardiac Sciences and is also a member of the Libin Cardiovascular Institute at the CSM.






